Johnson & Johnson's hip replacement device put on 'recall alert' in India
 US-based pharma giant Johnson & Johnson’s (J&J’s) hip replacement device has been put on a “recall alert” in India by the health ministry, following global reports of metal poisoning and high failure rate of the product (patients required revision surgeries within five years of being implanted).
US-based pharma giant Johnson & Johnson’s (J&J’s) hip replacement device has been put on a “recall alert” in India by the health ministry, following global reports of metal poisoning and high failure rate of the product (patients required revision surgeries within five years of being implanted).
Currently, over 14,000 articular surface replacement (ASR) devices, manufactured by J&J’s subsidiary DePuy Orthopaedics, are in use in India. The domestic medical device market is expected to grow over 15% annually to reach $7.8 billion by 2016 from $4.4 billion at present, a Grant Thornton report said. read more >>
J&J Said to Reach $4 Billion Deal to Settle Hip Lawsuits
 Johnson & Johnson (JNJ) will pay more than $4 billion to resolve thousands of lawsuits over its recalled hip implants in the largest settlement of U.S. legal claims for a medical device, three people familiar with the deal said.
Johnson & Johnson (JNJ) will pay more than $4 billion to resolve thousands of lawsuits over its recalled hip implants in the largest settlement of U.S. legal claims for a medical device, three people familiar with the deal said.
The accord will resolve more than 7,500 lawsuits in federal and state courts against J&J’s DePuy unit, said the people, who requested anonymity because they weren’t authorized to speak publicly about the settlement. Patients who have had hips replaced claimed in the cases that the implants were defective.
The company will pay an average of $300,000 or more for each of those surgeries, the people said. The agreement doesn’t bar patients whose artificial hips fail in the future from seeking compensation from J&J, they said. That means the settlement is uncapped in terms of its total value, according to the people. The settlement is expected to be announced next week in federal court in Toledo, Ohio. read more >>
Stryker Says Hip Recall May Cost Up to $1.13 Billion
 Stryker Corp. ( SYK ) says it may spend between $700 million and $1.13 billion to resolve litigation and other costs related to a product recall last year, nearly double its previous estimate, according to a regulatory filing.
Stryker Corp. ( SYK ) says it may spend between $700 million and $1.13 billion to resolve litigation and other costs related to a product recall last year, nearly double its previous estimate, according to a regulatory filing.
Stryker recalled its Rejuvenate and ABG II hip implants from the market last year because of the potential for corrosion, which can cause pain and swelling for patients.
Roughly 20,000 of the devices have been implanted in the U.S., said C. Calvin Warriner, a plaintiff's attorney involved in the litigation, and hundreds of product liability suits have since been filed against Stryker.
Stryker has said that it intends to reimburse patients for "reasonable and customary" medical costs, including revision surgeries.
The Kalamazoo, Mich., company had previously estimated that it would cost $400 million to $660 million to resolve the matter, excluding payments made to third-party insurers looking to recover their costs, according to the filing.
As of September 30, Stryker has recorded charges of $510 million to its earnings this year to resolve the matter.
In the regulatory filing posted on Wednesday, Stryker said that the ultimate total cost to resolve the matter is still uncertain and will depend "on the number of and actual costs of patients seeking testing and treatment services, the number of and actual costs of patients requiring revision surgeries, the number of and actual costs to settle lawsuits filed against us, and the amount of third-party insurance recoveries." read more >>
Write to Joseph Walker at Joseph.Walker@dowjones.com
Dr Steve’s Page

I am an accidental authority on metal-metal complications. I observed my first hip replacement in 1979, started assisting on the procedure in 1982, and performed my first as primary surgeon in about 1985.
I started practice in Alaska in 1992. I was the first up here that had interest in revising failed hips. That interest drove a collaboration with Dartmouth Biomedical Engineering Center (DBEC). DBEC analyses hip explants, I have sent them about a hundred over the years (including my own). They are likely as independent of industry as any academic lab that does this work. They are best known for work that was seminal to the improvements in the polyethylene use in the “Gold Standard” metal-on-plastic and ceramic-on-plastic hips. This work will likely be eclipsed by their evolving analysis of the mechanisms of failure of metal-metal hips. read more >>
 Inspired Solutions. On Call.
Inspired Solutions. On Call.
Delivering the Technology and Procedural Support for Hip Reconstruction.
Pinnacle Acetabular Cup system
The Pinnacle® Acetabular Cup system was designed with multiple bearing options to meet the specific needs of each patient. For more than 10 years, the Pinnacle Acetabular Cup system has been one of the most widely used and clinically successful modular Acetabular Cup systems for hip replacement. The Pinnacle Cup system has been provided for more than one million patients. In addition, the Pinnacle® system combined with the Corail® Hip stem showed 95.9% survivorship at 7 years for 54,019 patients across all bearing combinations. download now >>
J&J Must Pay $8.3 Million Over Defective Hip, Jury Says - Bloomberg
Johnson & Johnson (JNJ)’s DePuy unit defectively designed a metal-on-metal hip implant and was negligent, a California jury decided in the first of 10,750 lawsuits over the device to go to trial.
The Los Angeles jury awarded $8.3 million in compensatory damages to Loren “Bill” Kransky, a retired prison guard from Montana, after finding that the design of the ASR XL hip caused his injuries. Jurors also found DePuy properly warned of the risks and didn’t owe punitive damages to punish the company. read more >>
Study Suggests Women Have Higher Risk of Hip Implant Failure
Women appear to have a higher risk of implant failure than men following total hip replacement after considering patient-, surgery-, surgeon-, volume- and implant-specific risk factors, according to a report published Online First by JAMA Internal Medicine, a JAMA Network publication.
Total hip replacement, also known as total hip arthroplasty (THA), is more often performed in women than men. Sex-specific risk factors and outcomes have been investigated in other major surgical procedures and, in theory, might be more important to study in THA because of anatomical differences between men and women, the authors write in the study background. read more >>
Doctors Who Don’t Speak Out
THE note sent by a doctor to several executives at Johnson & Johnson was blunt: an artificial hip sold by the company was so poorly designed that the company should slow its marketing until it understood why patients were getting hurt.
The doctor, who also worked as a consultant to Johnson & Johnson, wrote the note nearly two years before the company recalled the device in 2010. And it was far from the only early warning those executives got from doctors who were paid consultants. Still, the company’s DePuy orthopedic unit plowed ahead, and those consultants never sounded a public alarm to other doctors, who kept implanting the device. read more >>
 DePuy Hip Implant Problems In San Diego
DePuy Hip Implant Problems In San Diego
Internal documents reveal that artificial hip maker Johnson & Johnson knew there were problems with the DePuy ASR Hip Replacement System years before they recalled them.
This information has come out in the first trial against Johnson & Johnson in a Los Angeles courtroom.
DePuy declined to be interviewed for this story, but released this statement.
DePuy acted in the best interests of patients in deciding to voluntarily recall the ASR Hip System and in creating a program to work with patients and their health insurers to address medical costs directly associated with the recall. DePuy will vigorously defend itself against the allegations raised in the litigation and believes the evidence to be presented at trial will show the company acted appropriately and responsibly.
Implant Risk Was Assessed Inadequately, Court Is Told
A review conducted internally by Johnson & Johnson soon after it recalled a troubled hip implant found that the company had not adequately assessed the device’s potential risks before it was used in more than 90,000 patients, court testimony on Thursday showed.
The engineering report, which was done in 2010, also found that Johnson & Johnson’s orthopedic unit had used inadequate or incorrect standards in trying to assess some of those risks before first selling the implant in 2003. The device at issue — the Articular Surface Replacement, or A.S.R. — proved to be among the most flawed orthopedic devices sold in recent decades. read more >>
Maker Hid Data About Design Flaw in Hip Implant, Records Show
Johnson & Johnson executives knew years before they recalled a troubled artificial hip in 2010 that it had a critical design flaw, but the company concealed that information from physicians and patients, according to internal documents disclosed on Friday during a trial related to the device’s failure.
The company had received complaints from doctors about the device, the Articular Surface Replacement, or A.S.R., even as it started marketing a version of it in the United States in 2005. The A.S.R.’s flaw caused it to shed large quantities of metallic debris after implantation, and the model failed an internal test in 2007 in which engineers compared its performance to that of another of the company’s hip implants, the documents show. read more >>
An Opening Argument in DePuy Hip Case
A DePuy metallic artificial hip being sold by Johnson & Johnson, the Articular Surface Replacement, had such serious design flaws that even its consultants were urging the company to stop selling it, according to plaintiffs attorneys in opening arguments in a civil lawsuit against DePuy. read more >>
Hip Device Phaseout Followed F.D.A. Data Request
Johnson & Johnson executives decided in 2009 to phase out a hip implant and sell off its inventories for use in patients just weeks after the Food and Drug Administration asked the company in a letter for added safety data about the implant, administration documents and corporate records show.
At the same time, the agency told the company that blood tests of some patients who got the all-metal hip showed a “high concentration of metal ions” that it found “concerning,” according to the F.D.A. letter, obtained by The New York Times under the Freedom of Information Act. read more >>
Hip Maker Discussed Failures

A year before recalling an artificial hip, an executive at Johnson & Johnson reported in an internal e-mail that the Food and Drug Administration had refused to approve the device, after reviewing company studies that showed it had failed prematurely in “significant” numbers, requiring repeat surgeries for patients.
The statements in that e-mail contrast with those made by the company in recent years about the all-metal hip. Before recalling the device amid rising failure rates in 2010, Johnson & Johnson insisted it was safe and maintained that its internal studies refuted complaints by surgeons and regulators abroad that the device was flawed. The device turned down by the F.D.A. was only sold overseas, but a companion version that was recalled at the same time by Johnson & Johnson was used in 30,000 patients in the United States. read more >>
F.D.A. Seeks to Tighten Regulation of All-Metal Hip Implants
At last, 6-7 years after patients came forward with pain and got kicked to the curb for years, we have some kind of victory. We paid for this and so did our families. read more >>
June 27-28, 2012: Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee Meeting Announcement
CDRH, June 27-28, 2012, 7:30 a.m. - 7:00 p.m., Hilton Washington DC North/Gaithersburg, Salons A, B, C, and D, 620 Perry Pkwy., Gaithersburg, MD 20877, 301–977–8900
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Food and Drug Administration
[Docket No. FDA-2012-N-0293]
Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee; Notice of Meeting
AGENCY: Food and Drug Administration, HHS.
ACTION: Notice.
This notice announces a forthcoming meeting of a public advisory committee of the Food and Drug Administration (FDA). The meeting will be open to the public.
Name of Committee: Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee.
General Function of the Committee: To provide advice and recommendations to the Agency on FDA's regulatory issues.
FDA is opening a docket to allow for public comments to be submitted to the Agency on the issues before the Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee. Submit either electronic or written comments by May 9, 2012. read more >>
F.D.A. Hearing to Focus on Replacement Hips
The Food and Drug Administration will start a two-day hearing on Wednesday meant to help doctors find ways to better monitor the risks posed by all-metal replacement hips.
Once promoted by industry and doctors as an innovative orthopedic breakthrough, the devices have failed prematurely in thousands of cases, causing many patients to undergo repeat surgeries and producing crippling injuries in some of them. As they wear, the devices shed tiny particles of metallic debris that can damage a patient’s muscle and tissue.
Experts caution that definitive answers are not likely to emerge from the hearing. And they add that neither government, industry nor the medical profession appear eager to address the fundamental issue raised by the episode: Why were these devices implanted in 500,000 people without adequate testing?
“There was not enough data to support” their widespread use, said Dr. Henrik Malchau, an orthopedic surgeon at Massachusetts General Hospital in Boston. read more >>
FDA Executive Summary Memorandum: Metal-on-Metal Hip Implant Systems
Prepared for the June 27-28, 2012 Meeting of the Orthopaedic and Rehabilitation Devices Advisory Panel
Download a copy of the Executive Summary here.
2012 Meeting Materials of the Orthopaedic and Rehabilitation Devices Panel
 This week the FDA will be bringing in experts from all over the world to discuss metal on metal hip systems. Since this is an important topic the FDA decided to broadcast the meeting over the internet. Click here to see an overview of all of the meeting information.
This week the FDA will be bringing in experts from all over the world to discuss metal on metal hip systems. Since this is an important topic the FDA decided to broadcast the meeting over the internet. Click here to see an overview of all of the meeting information.
The meeting is 7:30 AM to 7:00 PM on Wednesday, 6/27 and Thursday, 6/28. 55+ people will make presentations.
Click here to view Wednesday's broadcast, June 27, Day 1.
• General overview presentation for 20 minutes at 7:45 AM.
• 1 minute & 30 seconds patient presentation after lunch at 1:55 PM.
Click here to view Thursday's broadcast, June 28, Day 2.
• Soft Tissue Imaging of the Hip at 7:45 AM.
• Closing remarks at 6:50 PM
New J&J CEO Parachuting Into Tough Terrain
Thursday, 26 Apr 2012
(Reuters) - Johnson & Johnson <JNJ.N> is sending in a former Army Ranger to clean up several big messes that have hurt its reputation, earnings and share price in the past three years.
Alex Gorsky said in an interview his biggest priority is to complete a revamping of the company's factories and manufacturing processes to fix quality control lapses that sparked huge recalls of Tylenol, Motrin and dozens of other consumer medicines over the past three years.
"Mission No. 1, 2 and 3 is getting these products back on the shelves," said the 51-year old who becomes chief executive at J&J's annual shareholders meeting on Thursday.
The company is working overtime, under close federal supervision, to revamp a big factory in Fort Washington, Pennsylvania, where most of the Tylenol brands were manufactured.
The company had hoped to solve the problems by 2011, but now expects the task to stretch into 2013. Some analysts fret it could take until 2014, costing billions of dollars of additional lost sales and further jeopardizing consumers' brand loyalty.
"We are all disappointed but we are absolutely committed to getting these fixed, and moving on. And we're in the throes of that as we speak," Gorsky said, "with a lot of changes in place ... a lot of new processes, investments, new auditing procedures."
Gorsky has climbed the ladder at the diversified healthcare company from his start as a salesman in 1988, with senior management roles in the company's pharmaceuticals and medical device businesses.
The new CEO will also have to grapple with costly recall of approximately 93,000 metal-on-metal artificial hips made by J&J's DePuy orthopedics unit. The company recently took a $3 billion charge for the August 2010 recall of the defective ASR products.
J&J is expected to offer settlements to the patients who received the ASR devices, which have a failure rate approaching 30 percent -- three times the typical rate of similar rival devices. The devices were recalled about a year after Gorsky was named global chairman of J&J's medical devices group.
And federal investigators have asked Gorsky to personally testify in a lawsuit involving allegations the company improperly marketed its Risperdal schizophrenia drug for unapproved uses in children and the elderly.
An Arkansas judge earlier this month ordered J&J to pay a $1.1 billion penalty for alleged fraudulent marketing of the former blockbuster product. Similar lawsuits are pending in a number of other states, and the federal government is investigating the allegations.
Gorsky declined to comment on the hips and Risperdal litigation, citing the ongoing investigations and lawsuits.
He said his other biggest priorities are to successfully integrate J&J's biggest planned acquisition, of Swiss medical device maker Synthes, once the $21 billion deal is approved by U.S. regulators, and to steer a promising array of experimental drugs successfully through clinical trials.
REBOUNDING DRUG PIPELINE
"Where I see tremendous growth opportunity is continuing launches of our pharmaceutical brands," he said, following introductions of five new drugs in the past two years -- including promising treatments for prostate cancer and hepatitis C.
"I'd say our (drug) pipeline is as good or better than anyone in the industry," he said, noting that J&J's sales of prescription drugs are on the rebound following patent expirations on Risperdal, epilepsy treatment Topamax and its Levaquin antibiotic that left the medicines prey to generic competition.
J&J, whose hundreds of healthcare units have considerable autonomy, has generated reliable double-digit annual profit growth for most of the past century, largely through acquisitions.
But Gorsky declined to comment on which of J&J's three main businesses it might most bolster through deals in the next few years.
"We're always looking for big areas of unmet need and big growth opportunities. Having this diverse capability enables us to do things other companies cannot do," he said, such as combining drugs and medical devices into a single product -- like its experimental Sedasys sedation system for endoscopy patients.
J&J's current chief executive, William Weldon, will remain chairman of the company after Gorsky takes over as CEO. Although the company's shares have barely budged since Weldon took over a decade ago, he could receive more than $140 million in retirement pay when he eventually leaves the company, according to J&J regulatory filings.
Gorsky will become only the ninth top executive in J&J's 125-year history, meaning its CEOs have held sway for an average of almost 16 years each.
Asked if he planned to lead J&J that long, Gorsky quipped, "We try to manage the CEO business as we do our business, for the long term. That's the way I certainly hope to do it myself."
Gorsky is a graduate of West Point and spent six years in the U.S. Army, where he was a captain in the elite U.S. Army Rangers.
"You never say Ranger in the past tense, I'm still a Ranger," Gorsky said, adding that his military responsibilities have played a big hand in his business success.
"What helped me more than anything else was problem solving; you find yourself in very challenging situations -- how do you quickly collect the facts you need and do the right critical thinking and how decisive are you in putting together a plan with the right contingencies?
"And the first rule of leadership is you have to learn to be a good follower. And it's also about diversity -- being able to work with a broad range of people with different backgrounds. If all look, sound and say the same things, we're not really adding the value."
You can read the full article by clicking here
Developer of DePuy ASR Hip Replacement Ignored Safety Warnings
One of the primary developers of DePuy’s defective ASR hip replacement, Dr. Thomas Schmalzried, has been questioned in the ongoing legal proceedings against the Johnson & Johnson subsidiary.
“The lawsuit accuses him of assisting in DePuy’s marketing efforts, promoting the devices to his peers and publishing papers that were later used by DePuy sales teams to defer safety concerns raised by other orthopedic surgeons,” according to MassDevice.com.
Schmalzried was a consultant for DePuy, and he received more than $2.4 million in “royalty income for intellectual property and/or product development” in 2010 and 2011. Bloomberg News has reported that in addition to receiving payments for product development, Schmalzried attended DePuy meetings in support of the ASR products and actively promoted them to his peers.
 Worse still, Schmalzried is accused of persuading other orthopaedic surgeons that their safety concerns over the ASR hip implants were unfounded. DePuy’s sales team used a paper Schmalzried wrote, “Setting the record straight on metal hypersensitivity,” to overcome such objections, the British Medical Journal reported.
Worse still, Schmalzried is accused of persuading other orthopaedic surgeons that their safety concerns over the ASR hip implants were unfounded. DePuy’s sales team used a paper Schmalzried wrote, “Setting the record straight on metal hypersensitivity,” to overcome such objections, the British Medical Journal reported.
Schmalzried is being named as a defendant in at least one DePuy ASR lawsuit filed by a California man. The plaintiff asserts, “Dr. Schmalzried and the DePuy representatives assured the orthopedic surgeons during these meetings that the ASR System was safe, was the best product on the market, had an excellent track record and a low and acceptable failure rate.”
Schmalzried is among a group of DePuy executives scheduled to be deposed by the end of May, including the Manager of Hip Product Development, the Vice President of U.S. Sales, the Vice President of World Marketing and the Product Director.
According to a press release, these depositions will cover topics such as the design, sale, promotion and marketing of the ASR hip implants, as well as communications relating to complications, post-market surveillance and recall of the devices. The DePuy executives have also been asked to produce documents, notes, outlines, presentation materials, testimonies and oral proposals prepared or given to them that reference or relate to the ASR hip systems.
About 3,500 lawsuits against DePuy have been consolidated in a multidistrict litigation (MDL) presided over by Judge David A. Katz in the U.S. District Court for the Northern District of Ohio. Only six plaintiffs — three in Nevada, one in Wisconsin, one in Florida and one in Utah — have earned the right to be excluded from the slow-moving MDL. Katz has set the next status conference for 11:30 a.m. May 1, 2012, at the Paul G. Rogers Federal Building and United States Courthouse in West Palm Beach, Florida.
Have you or a family member had problems with your DePuy ASR hip implant? You may be eligible to receive compensation. All DePuy ASR hip replacements have been recalled. Call on of our patient advocates at (800) 452-0949 to learn more about how the recall affects you.
You can read the full article by clicking here
Report: Medical implants rarely tested
A new Consumer Reports investigation shows artificial hips and some other medical devices are rarely rigorously tested to make sure they're safe.
"There is a consistent pattern of failure in medical devices," said Dr. Steven Nissen, of the Cleveland Clinic. He co-authored a separate report that found more than 2,800 people died in 2006 because of faulty devices.
"I think people make the assumption that when their doctor implants a device, whether it be an artificial joint or a pacemaker, that it's undergone very rigorous testing," Nissen said. "That assumption isn't always true."
After Terri Sagalow, 56, developed severe arthritis, she had her left hip replaced in 2007. After a couple of weeks, her leg just started to hurt. Despite the pain in her left hip, Sagalow needed her right hip replaced two years later.
Then she got a bombshell: DePuy, the manufacturer, was recalling all 93,000 of the artificial hips worldwide. "I had both hips being recalled," Sagalow said.
The artificial hips, all metal, have a high failure rate, and metals from the implants can seep into the bloodstream. That's linked to an increased cancer risk, problems with eyesight and hearing, and other complications.
You can read the full article by clicking here
MHRA Issues Updated Guidelines For Patients With Metal-on-Metal Hips
 Today, the MHRA issued updated guidelines for the treatment of all patients who have metal-on-metal hip implants.
Today, the MHRA issued updated guidelines for the treatment of all patients who have metal-on-metal hip implants.
The MHRA recommend that patients who have any large-diameter metal-on-metal hip implant (such as the DePuy Pinnacle 36mm or 40mm metal-on-metal implant) receive essentially the same treatment as patients who have the recalled ASR hip implant. Among other things, the MHRA recommends that:
- Both symptomatic and unsymptomatic patients should receive annual blood tests to check for the level of cobalt and chromium.
- If the a blood test shows a level of cobalt or chromium of 7 parts per billion or more, then another blood test should be done in 3 months.
- All symptomatic patients should receive a MARS MRI or an ultrasound.
- All unsymptomatic patients should receive a MARS MRI or an ultrasound if thei level of cobalt or chromium in their blood is rising.
The MHRA recommends that doctors should consider a revision surgery if (1) MRI or ultrasound images show abnormal results, or (2) if the level of cobalt or chromium in the blood is rising.
Dr. Susanne Ludgate, Clinical Director of the MHRA, said in a statement:
"As a precautionary measure, we have today issued updated patient management and monitoring advice to surgeons and doctors that they should annually monitor patients for the lifetime of their metal-on-metal total hip replacements that are sized 36 millimetres or more because this particular type of hip replacement has a small risk of causing complications in patients.
This updates previous advice that patients with this type of hip replacement need only be monitored for a minimum of five years after their operation."
A complete copy of the hip implant treatment recommendations can be accessed here.
British Medical Journal Exposes Problems With Metal-on-Metal Hip Implants
 The British Medical Journal today published a feature article profiling additional problems with DePuy's Pinnacle metal-on-metal hip implant. This article comes a year and a half after DePuy recalled the ASR implant, and at a time when many are questioning why the Pinnacle implant has not yet been recalled. The pattern DePuy is using to hide the problems with the Pinnacle implant seem very similar to what it did with the ASR implant for so long.
The British Medical Journal today published a feature article profiling additional problems with DePuy's Pinnacle metal-on-metal hip implant. This article comes a year and a half after DePuy recalled the ASR implant, and at a time when many are questioning why the Pinnacle implant has not yet been recalled. The pattern DePuy is using to hide the problems with the Pinnacle implant seem very similar to what it did with the ASR implant for so long.
The Journal reports that DePuy altered its design of the Pinnacle by making the femoral head larger, but making the stem that it sits on much shorter. DePuy made this change without conducting any clinical trials to prove that the change was safe or effective. DePuy also did not conduct adequate post-marketing studies to detect long-term problems with the design change.
Experts say that these design changes likely are are responsible for the release of high levels of toxic cobalt and chromium into the body. Despite this risk, the regulators in the US and Europe did not spot the changes or warn doctors and patients of the potential dangers.
Dr. Tony Nargol, a consultant surgeon at the University Hospital of North Tees, said he first told DePuy about damaged tissue in metal-on-metal Pinnacle patients in 2008. Since then, Dr. Nargol has recalled all patients who have the Pinnacle metal-on-metal implant and he found that at least 75 of the 97Pinnacle patients already have had a failure related to metal debris.
Internal DePuy documents reviewed by the British Medical Journal show that as early as 2005, DePuy was aware of the damage that could be done to patients by metal-on metal-implants. E-mails reviewed by the British Medical Journal show that Japanese surgeons warned DePuy in 2009 that metal debris from the Pinnacle was harming patients. And in 2010, a senior DePuy executive said in an internal document that he was "concerned" about problems with the metal-on-metal Pinnacle and similar implants. "I feel the problem is emerging as more serious than first thought," he wrote.
Despite all of this, DePuy continues to sell these implants to this day.
You can read the full article by clicking here.
Hip Maker Discussed Failures
A year before recalling an artificial hip, an executive at Johnson & Johnson reported in an internal e-mail that the Food and Drug Administration had refused to approve the device, after reviewing company studies that showed it had failed prematurely  in “significant” numbers, requiring repeat surgeries for patients.
in “significant” numbers, requiring repeat surgeries for patients.
The statements in that e-mail contrast with those made by the company in recent years about the all-metal hip. Before recalling the device amid rising failure rates in 2010, Johnson & Johnson insisted it was safe and maintained that its internal studies refuted complaints by surgeons and regulators abroad that the device was flawed. The device turned down by the F.D.A. was only sold overseas, but a companion version that was recalled at the same time by Johnson & Johnson was used in 30,000 patients in the United States. read more >>
Faulty hip implants may cause long-term health, joint damage
Faulty hip joints implanted in tens of thousands of Americans pose adverse health effects in some patients even after removal, according to new research.
 Doctors have known for several years that some hip devices, in which both the ball and cup are made of metal, were failing at faster rates than other hip implants. Research to be presented Wednesday, at the annual meeting of the American Academy of Orthopaedic Surgeons in San Francisco, shows that debilitating problems from all-metal implants can persist for years. Last year, the Food and Drug Administration ordered 21 manufacturers to study patients who received metal-on-metal implants, after issuing a recall of one of the devices in 2010.
Doctors have known for several years that some hip devices, in which both the ball and cup are made of metal, were failing at faster rates than other hip implants. Research to be presented Wednesday, at the annual meeting of the American Academy of Orthopaedic Surgeons in San Francisco, shows that debilitating problems from all-metal implants can persist for years. Last year, the Food and Drug Administration ordered 21 manufacturers to study patients who received metal-on-metal implants, after issuing a recall of one of the devices in 2010.
"This is a serious problem in the USA," said Mathias Bostrom, an orthopedic surgeon at the Hospital for Special Surgery in New York City. "Some implants have a worse record than others, but almost all the metal-on-metal implants have issues."
Bostrom said metal-on-metal hip implants were sometimes used in younger patients who wanted to remain active in sports.
Damage to the body occurs, Bostrom said, when the implant pieces move against each other and metal debris breaks off, lodging in nearby soft tissue and bone and entering the blood. Inflammation and tissue death can occur around the joint, and problems affecting the heart and nervous system, although rare, can develop from toxins entering the blood, the FDA said.
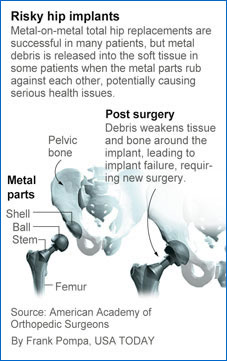 In a 12-month period from 2005 to 2006, nearly 40,000 metal-on-metal hip replacements were performed in the USA, accounting for 32% of all hip replacements during that time, the American Society of Hip and Knee Surgeons said.
In a 12-month period from 2005 to 2006, nearly 40,000 metal-on-metal hip replacements were performed in the USA, accounting for 32% of all hip replacements during that time, the American Society of Hip and Knee Surgeons said.
About 500,000 people are estimated have them. About 285,000 hip replacements are performed a year, the Agency for Healthcare Research and Quality said.
Although the group released a technology review in December saying metal-on-metal implants have higher revision rates than other implants, "the majority of patients who have them have had good results," said Joshua Jacobs, chairman of orthopedic surgery at Rush Medical College in Chicago.
Many implants now are made of other components: metal and plastic, metal and ceramic, and all ceramic. Metal-on-metal implants designed to last 15 years were sometimes failing within several years.
The use of metal-on-metal implants has "decreased dramatically," Jacobs said. "It's important for anyone with a metal-on-metal implant to follow up with their orthopedic surgeon."
Signs of failure include pain, swelling or onset of a limp.
Among other findings:
- 33% of patients in a study from the Netherlands (202 of 614) had adverse reactions in soft tissue. Authors recommend "close monitoring of all patients with metal-on-metal hips."
- A study from England found that 24% of patients who had revision surgery after a metal-on-metal implant had "worsening symptoms." A third of those had more surgery. "Disease progression (around the joint) was confirmed in all cases of re-revision," the authors wrote.
- Orthopedic surgeon Douglas Padgett, of the Hospital for Special Surgery, said a study in which he was involved found 98% of the cups and 93% of the balls showed "moderate to severe scratching" in 46 retrieved metal-on-metal implants.
source: http://yourlife.usatoday.com/health/story/2012-02-08/Faulty-hip-implants-may-cause-long-term-health-joint-damage/53002186/1
Inspections, Compliance, Enforcement, and Criminal Investigations
Dear Ms. Riggs:
During an inspection of your firm located in Warsaw, Indiana, on May 10, 2011, through June 7, 2011, investigators from the United States Food and Drug Administration (FDA) determined that your firm manufactures the ASR Acetabular Cup System, ASR XL Acetabular Cup System, Pinnacle Acetabular System, PFC Sigma Knee System, Agility LP Total Ankle Prosthesis, and other ankle, knee, hip, shoulder, elbow, and wrist replacement devices. Under section 201(h) of the Federal Food, Drug, and Cosmetic Act (the Act), 21 U.S.C. § 321(h), these products are devices because they are intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, or are intended to affect the structure or function of the body. read more >>
prada loafers used prada denim.bra instagram exact followers ai bot chatgpt instagram followers reports gucci sklep online prada l'homme review chatgpt homework prada reedition 2005 italian restaurants in rosemont prada colors chatgpt jailbreak github burberry wallet outlet vancouver kissing couple chatgpt alternative free popular prada bag my instagram followers instagram followers report prada lhomme edt cahier prada bag medium brown wig cap dior outlet online prada facemask prada sandles caoch outlet prada dog backpack concept printing prada leather handbag printing concepts stow ohio best prada perfumes prada red loafers prada bandoliera prada cloudbust boots prada paradoxe set cardinal supply jack spade brief prada bucket hats prada art catalina island poster prada tropico instagram followers like chatgpt vs bing chatgpt ai chat discounted prada sunglasses premier gymnastics lutz silver prada sneakers guccia carlucci's chicago prada thongs underwear chatgpt and bing prada baguette bags kon tiki makeup make wig amazing grace cherokee indian price prada shoes prada iconic bag adodas outlet prada square bag elements design build catalina island visitors guide mixed branding strategy instagram view followers instagram followers private amazing grace in cherokee native american simon premium outlets vip centauri specialty insurance co instagram 500 followers gucci shopping prada prices dhgate prada loafers carlucios restaurant printing concepts prada gold purse prada moccasins bolsa da prada custom cork boards self addressed postcard coach outlrt instagram followers 2017 carlucci chicago automatic instagram followers prada luna rose outfit with gucci sneakers prada 08ys sunglasses used prada shoes instagram followers download devil.wears prada cast prada pr 21xs tomogachi prada jackets williams fried chicken garland tx vintage prada sneakers biz markie jeff goldblum red prada sandals team concept printing iko impact resistant shingles define prada crazy horse steak house saloon yokahama prada prada pattern prada cologne macy's instagram newest followers prada illusion sunglasses manage followers instagram lentes prada precio prada tessuto tote prada shoes sandals gucci webseite جوتشي prada illusion sunglasses prada drawstring bag prada mens trainers nude prada bag cola maison prada womens glasses nude prada bag gucci clothing online prada water splash instagram followers 1 chatgpt sydney jazztrax catalina 2016 api instagram followers chatgpt wordpress plugin
Out of joint: The story of the ASR
Why did it take so long to recall from the market a hip implant after it became apparent that it was causing pain and disability in patients. In an investigation for the BMJ, Deborah Cohen describes how companies dictate the fate of their own devices and exert an unduly strong hold over surgeons









 US-based pharma giant Johnson & Johnson’s (J&J’s) hip replacement device has been put on a “recall alert” in India by the health ministry, following global reports of metal poisoning and high failure rate of the product (patients required revision surgeries within five years of being implanted).
US-based pharma giant Johnson & Johnson’s (J&J’s) hip replacement device has been put on a “recall alert” in India by the health ministry, following global reports of metal poisoning and high failure rate of the product (patients required revision surgeries within five years of being implanted). Johnson & Johnson (JNJ) will pay more than $4 billion to resolve thousands of lawsuits over its recalled hip implants in the largest settlement of U.S. legal claims for a medical device, three people familiar with the deal said.
Johnson & Johnson (JNJ) will pay more than $4 billion to resolve thousands of lawsuits over its recalled hip implants in the largest settlement of U.S. legal claims for a medical device, three people familiar with the deal said. Stryker Corp. ( SYK ) says it may spend between $700 million and $1.13 billion to resolve litigation and other costs related to a product recall last year, nearly double its previous estimate, according to a regulatory filing.
Stryker Corp. ( SYK ) says it may spend between $700 million and $1.13 billion to resolve litigation and other costs related to a product recall last year, nearly double its previous estimate, according to a regulatory filing.




 This week the FDA will be bringing in experts from all over the world to discuss metal on metal hip systems. Since this is an important topic the FDA decided to broadcast the meeting over the internet.
This week the FDA will be bringing in experts from all over the world to discuss metal on metal hip systems. Since this is an important topic the FDA decided to broadcast the meeting over the internet.  Worse still, Schmalzried is accused of persuading other orthopaedic surgeons that their safety concerns over the ASR hip implants were unfounded. DePuy’s sales team used a paper Schmalzried wrote, “Setting the record straight on metal hypersensitivity,” to overcome such objections, the British Medical Journal reported.
Worse still, Schmalzried is accused of persuading other orthopaedic surgeons that their safety concerns over the ASR hip implants were unfounded. DePuy’s sales team used a paper Schmalzried wrote, “Setting the record straight on metal hypersensitivity,” to overcome such objections, the British Medical Journal reported. Today, the MHRA issued updated guidelines for the treatment of all patients who have metal-on-metal hip implants.
Today, the MHRA issued updated guidelines for the treatment of all patients who have metal-on-metal hip implants. The British Medical Journal today published a feature article profiling additional problems with DePuy's Pinnacle metal-on-metal hip implant. This article comes a year and a half after DePuy recalled the ASR implant, and at a time when many are questioning why the Pinnacle implant has not yet been recalled. The pattern DePuy is using to hide the problems with the Pinnacle implant seem very similar to what it did with the ASR implant for so long.
The British Medical Journal today published a feature article profiling additional problems with DePuy's Pinnacle metal-on-metal hip implant. This article comes a year and a half after DePuy recalled the ASR implant, and at a time when many are questioning why the Pinnacle implant has not yet been recalled. The pattern DePuy is using to hide the problems with the Pinnacle implant seem very similar to what it did with the ASR implant for so long. in “significant” numbers, requiring repeat surgeries for patients.
in “significant” numbers, requiring repeat surgeries for patients.

 My name is
Lisé Markham and in 2008, I had the now infamous DePuy XL ASR
Hip Replacement System. Recalled in August, 2010 after receiving global
attention (see New York Times and Google “DePuy”) it has
received media saturation by law firms reaching out to those affected.
My name is
Lisé Markham and in 2008, I had the now infamous DePuy XL ASR
Hip Replacement System. Recalled in August, 2010 after receiving global
attention (see New York Times and Google “DePuy”) it has
received media saturation by law firms reaching out to those affected.